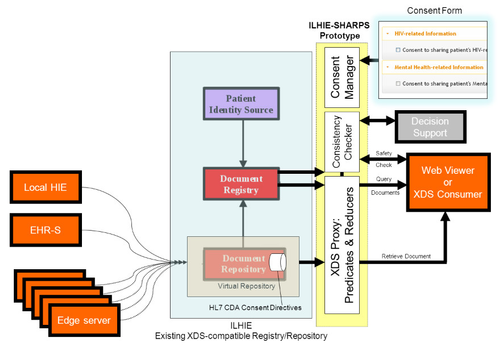
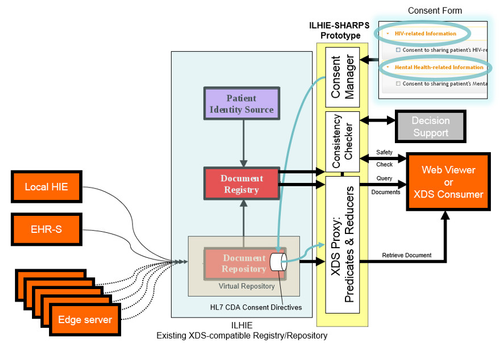
HIE Prototype Architecture
When installed in front of an HIE’s XDS Registry/Repository service, and configured to intercept its inbound XDS requests, the prototype queries for a patient consent directive in the repository, and sends it – along with the CCD returned by the HIE – to the Predicate/Reducer. The prototype then returns the redacted CCD to the requester, as illustrated below.
| HIE Prototype – architecture diagram | HIE Prototype – architecture diagram showing flow of consent directive |
The overall HIE architecture, which is common among state HIEs and implemented by a number of commercial vendors, involves provider EHRs connected to a central HIE registry, directly or via proprietary “edge servers.” The HIE maintains a document registry of patient CCDs, either stored in a central repository or in federated edge servers, and responds to requests for documents from “consumers” (typically clinicians who are participants in the HIE) running XDS client software in an EHR or using a web-based XDS viewer.
After the redacted CCD is returned by the prototype, the requester – knowing that the CCD may have been redacted – can initiate a safety check. Drug-drug interaction safety checking, part of a more generic idea called “Consistency Checking,” is designed to help mitigate the potential negative impact of redacted data on patient care. The prototype drug interaction safety checking service is a web-based application that responds (with a true or false result) to requests to compare a proposed medication list with all known interactions with medications in the non-redacted CCD. The prototype also includes a simple web application to create patient consent directive documents to be used by the predicate reducer.
For each of the three prototype components – Predicate/Reducer proxy, Safety Checker, and Consent Form – the scope of the prototype is limited for demonstration purposes but the architecture is extensible so that the scope can be expanded in the future. For example, the safety checking is limited to medications but could potentially cover additional clinical domains beyond medications. Consent directives supported by the prototype are relatively simple and are limited to indicate a preference for disclosure or non-disclosure of specific conditions such as HIV and mental health information.